Drug Repositioning
Drug innovation is a time consuming (it takes typically 12-14 years), expensive (ca. 2.5 billion USD) and risky business (failure rates are over 90%, due mainly to safety and efficacy issues).
Among new strategies considered to improve the output of de novo drug innovation, drug repositioning, also known as drug repurposing or re-profiling, based on the utilization of an approved drug for a new therapeutic application, has recently attracted considerable attention. It represents a relatively short, less expensive, successful path to introduction of a new therapy in healthcare. Although most examples of repositioning were born on a serendipity basis, currently intense work is being done to place repositioning on a rational basis.
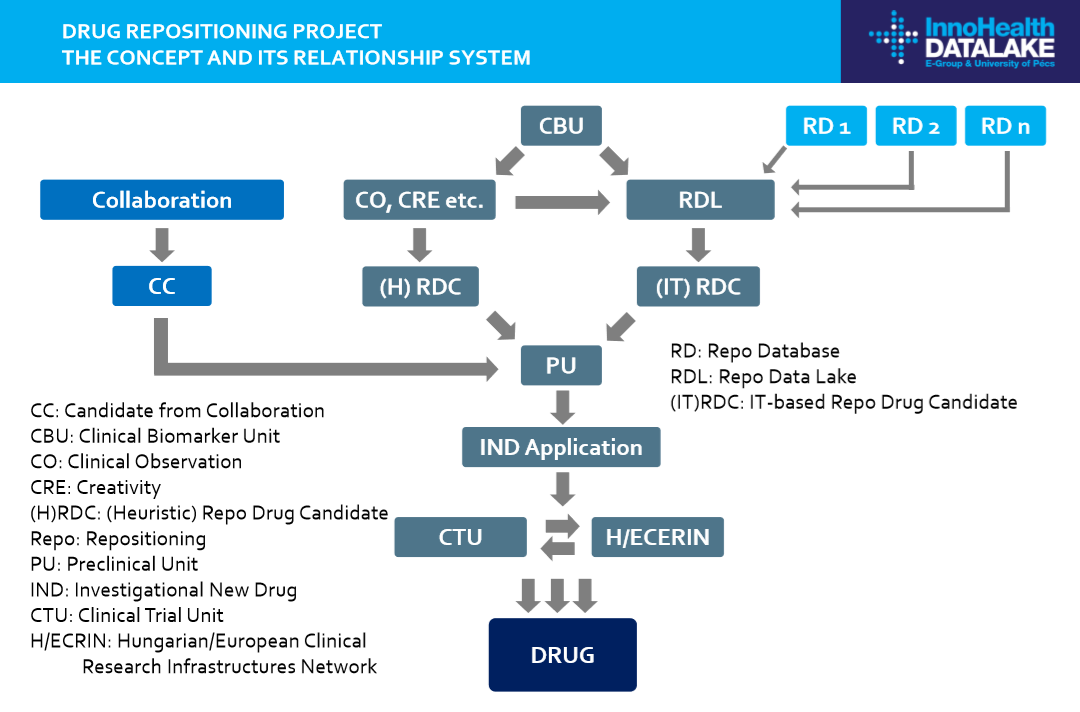
In our project, we create an IT platform (‘Repo DataLake’) with all relevant data to help identify new candidate molecules through data analytics. In addition, heuristic-based solutions are also implemented in the system. We believe that with the synergy of the two approaches we can identify new valuable candidates for repositioning (Figure), which are evaluated in preclinical and clinical phases.
Moreover, we also expect other important results within the framework of the project, such as identification of new biomarkers and comorbid diseases.
Another aspect of the project is to monitor and re-evaluate the clinical efficacy and safety of selected drugs based on real world data. It can reposition the drugs from cost-effectiveness and therapeutic profile considerations to control and optimize its position in healthcare system.
Participants:
RepoLake (IT Platform):
- Prof. Péter Mátyus (E-Group)
- Márton Perényi (E-Group)
- Prof. Lajos Botz (PU)
Real World Repo:
- Prof. Zsuzsanna Helyes (PU)
- Prof. Tamás Kálai (PU)
- Anita Steib (PU)
- Viktória Gaszner-Kormos (PU)
- Szilárd Pál (PU)
- Aleksandar Széchenyi (PU)
- Prof. Péter Mátyus (E-Group)
InnoHealth Datalake
Modules


